If you’re searching for a sterile, pharmaceutical-grade solution to reconstitute powdered compounds, you’ve come to the right place. We supply high-purity Bacteriostatic Water (Bac Water) and related aqueous diluents that meet the rigorous standards of the United States Pharmacopeia (USP). Whether you’re working in a compounding pharmacy, biomedical lab, or clinical research facility, our sterile solutions are designed to support your precision workflows.
We proudly offer fast, secure shipping throughout Pennsylvania. Our streamlined ordering process ensures your supplies arrive promptly, so you can focus on what matters most: safe, effective preparation.
When working with biologically sensitive materials, the sterility and consistency of your diluent are critical. Our Bacteriostatic Water is formulated to support multi-use applications while maintaining microbial control and compound integrity
Manufactured as Sterile Water for Compounding, our Bac Water includes a preservative and undergoes rigorous quality checks to meet pharmaceutical-grade standards.
Each vial contains 0.9% Benzyl Alcohol (BnOH), a bacteriostatic agent that inhibits bacterial growth after the vial is accessed—making it suitable for repeated withdrawals.
Our logistics are tailored to meet the needs of customers across the state, ensuring fast processing and dependable delivery.
Available in convenient sets such as 3-vial and 10-vial packs, allowing you to choose the quantity that best fits your reconstitution and storage protocols.
Bacteriostatic Water is a sterile aqueous solution that contains benzyl alcohol preservative that prevents bacterial growth. Unlike bactericidal agents that kill bacteria outright, bacteriostatic compounds inhibit their multiplication, making Bac Water ideal for multi-use scenarios.
This preservative feature allows Bac Water to remain viable across multiple withdrawals, provided aseptic technique is maintained. It’s especially useful when reconstituting powdered substances that are administered over time, such as in multi-dose formulations or ongoing research protocols.
Selecting the correct solution is essential for maintaining the stability and safety of your reconstituted compound. While both Sterile Water and Bacteriostatic Water are used in pharmaceutical and research settings, they differ in composition and intended use.
This is pure, pyrogen-free H₂O without any preservatives or buffering agents. It’s suitable for single-use applications where the entire contents are consumed immediately after reconstitution. Any remaining solution must be discarded to avoid contamination.
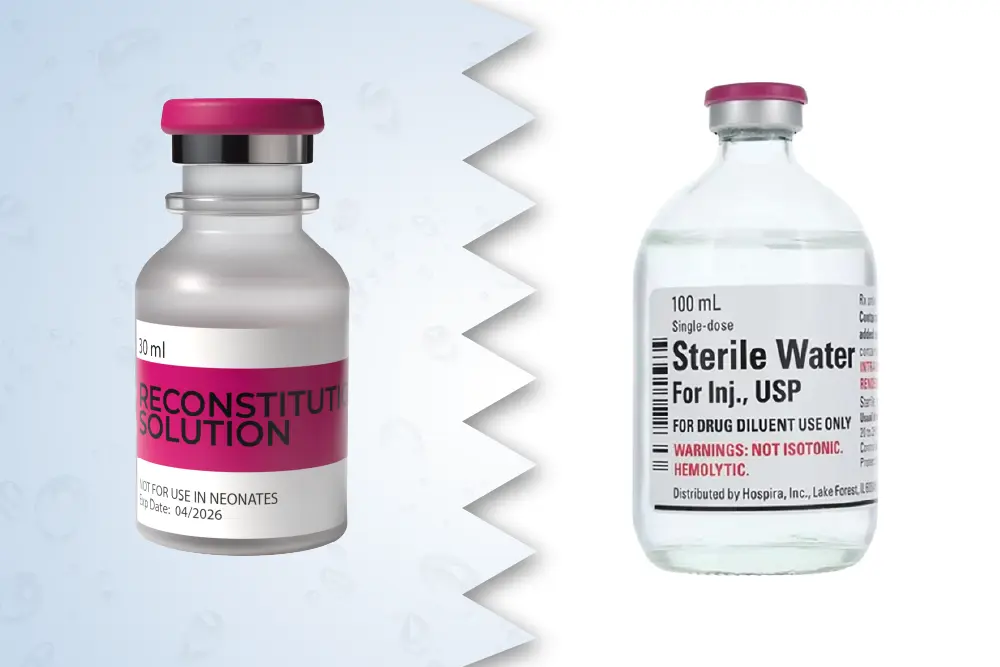
Contains 0.9% Benzyl Alcohol, which inhibits bacterial growth after the vial is opened. This allows for multiple withdrawals over a defined period, making it ideal for compounds that require repeated dosing. Always verify compatibility with your specific compound by consulting manufacturer guidelines
When using Bac Water, proper technique is essential to maintain sterility. This process, known as Aseptic Reconstitution, requires a clean workspace, sterile materials (syringes, needles, alcohol pads), and strict attention to detail. To reconstitute, simply sanitize the rubber stoppers of both the Bac Water and the compound vial. After withdrawing the specified volume of Bac Water, inject it slowly into the powdered compound vial, aiming the stream against the side of the glass to prevent foaming. Gently swirl the vial until the powder is fully dissolved (do not shake). Finally, label the reconstituted vial clearly with the compound name, concentration, the date of preparation, and the new expiration date (following the manufacturer’s guidelines).
Proper technique is essential when using Bac Water to reconstitute powdered compounds. Aseptic reconstitution minimizes the risk of contamination and ensures the integrity of your final solution.
Here’s a recommended procedure:
Use a clean, disinfected surface and ensure all materials are sterile.
Clean the rubber stoppers of both the Bac Water and compound vial using alcohol swabs
Employ sterile syringes and needles for all transfers.
Draw the required volume of Bac Water and inject it slowly into the compound vial. Aim the stream against the inner wall of the vial to prevent foaming.
Swirl the vial gently until the powder is fully dissolved. Avoid shaking, which can denature sensitive compounds.
Mark the reconstituted vial with the compound name, concentration, preparation date, and updated expiration date based on manufacturer instructions.
Our high-quality Bacteriostatic Water is a foundational component in numerous sensitive procedures, providing the highest standard of aqueous dilution.
Used in the preparation of multi-dose sterile formulations, including peptides, biologics, and lyophilized drugs. Bac Water helps maintain concentration and stability throughout the dosing cycle
Ideal for preparing reagents, diluting samples, and reconstituting proteins and peptides for bioassays, cellular studies, and molecular screening.
Suitable for creating sterile solutions for animal care, niche therapeutics, and compounded products requiring repeated access.
Used in media preparation and contamination checks for industrial and cosmetic products, where microbial control is essential.
In high-tech industries such as semiconductors and aerospace, sterile water solutions are used for cleaning and lubrication processes that demand zero contamination.
We understand that timely delivery is essential for maintaining operational efficiency. That’s why we’ve optimized our ordering and shipping process to serve customers throughout Pennsylvania.

Looking for high-quality bacteriostatic products in your area? We provide sterile aqueous solutions designed for safe and effective use in research, compounding, and specialized applications.
🔗 Visit us online https://universal-solvent.com/shop-2/
to explore our full product range and place your order today.